Latest News
January 1, Supreme Court Verdict Looms on Mystery Pill
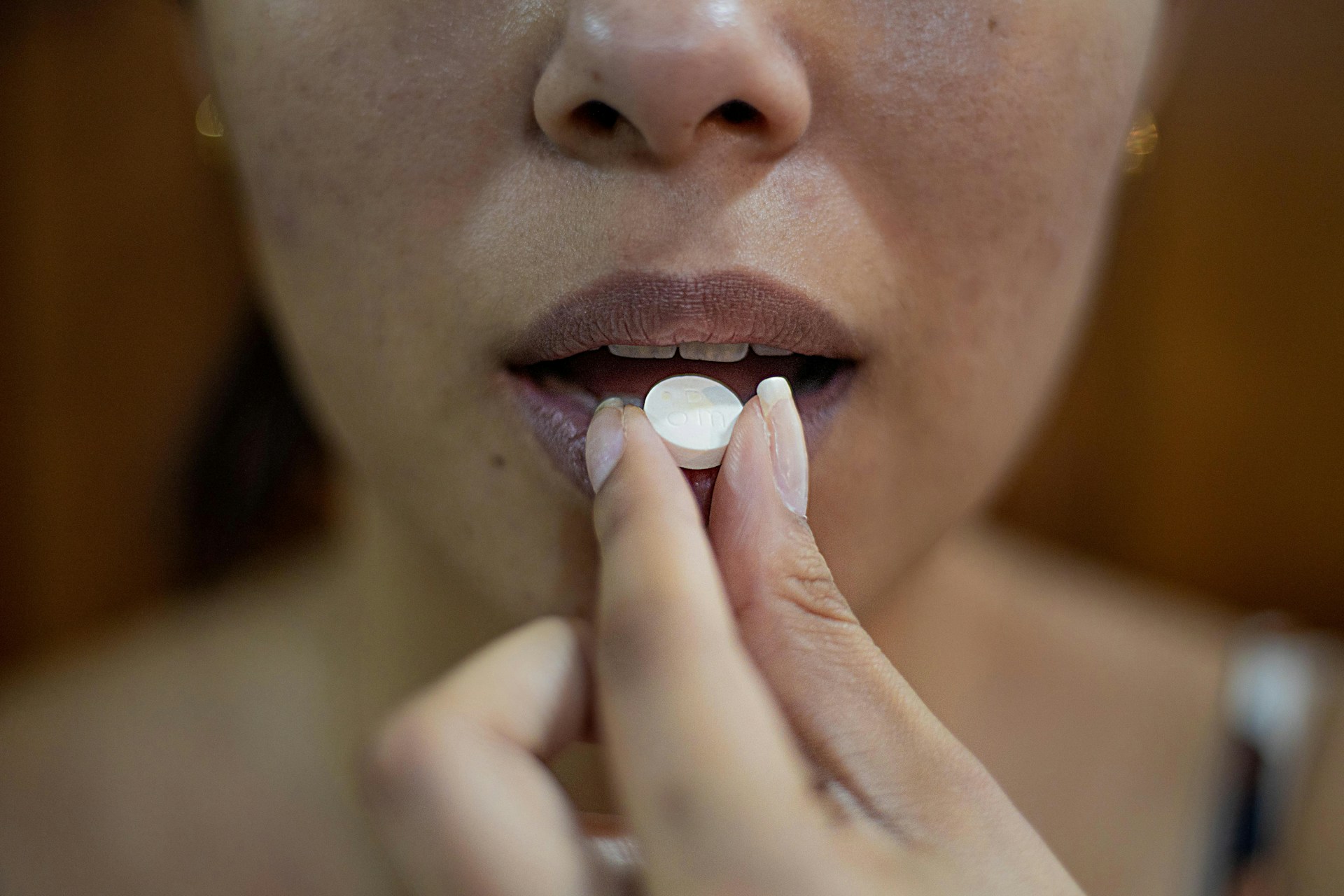
The Supreme Court is preparing to hear a significant challenge to the abortion pill, brought forth by doctors and medical associations. The case, FDA v. Alliance for Hippocratic Medicine, is one of two abortion-related cases the Supreme Court will hear this term since the overturning of Roe v. Wade in 2022. The justices will examine the U.S. Food and Drug Administration’s (FDA) decision to roll back safety regulations for the chemical abortion drug mifepristone.
Doctors who have sued the FDA argue that the removal of safety standards, once considered “essential,” increases the likelihood of women needing medical treatment. This forces OB-GYNs and emergency room doctors to deal with the “serious complications caused by these drugs.”
Dr. Ingrid Skop, a member of the plaintiff organization American Association of Pro-Life Obstetricians and Gynecologists (AAPLOG), expressed her concerns in a declaration, stating, “My moral and ethical obligation to my patients is to promote human life and health. But the FDA’s actions may force me to end the life of a human being in the womb for no medical reason.”
In 2021, the FDA permitted the distribution of mifepristone through the mail and eliminated the requirement for an initial in-person visit. Earlier, in 2016, the agency removed many of the safeguards implemented when the pill was approved in 2000, allowing it to be used through ten weeks of pregnancy, rather than seven.
Katie Glenn Daniel, state policy director for Susan B. Anthony Pro-Life America, expressed concern over the FDA’s actions, stating, “A lot of people have no idea that under Joe Biden’s [FDA], abortion drugs can be sent through the mail to their kids, even if they live in a pro-life state.”
If the Supreme Court sides with the doctors, it would mean a return to pre-2016 regulations on mifepristone while the lawsuit continues in the lower courts. The initial challenge was brought in 2022 by four medical associations and four individual doctors, alleging the FDA’s relaxed rules put women’s lives at risk and harm doctors in various ways.
In addition to challenging the removal of safeguards, they argued the FDA’s rapid approval of the drug was flawed from the start, requiring the agency to designate pregnancy as an “illness” and the pill as providing a “meaningful therapeutic benefit.”
The Supreme Court will consider three questions: whether the doctors have standing to bring the case, the FDA’s decision to roll back safety regulations after 2016, and the preliminary injunction granted by the lower courts.
In April 2023, a district court judge ruled that the FDA must reverse its approval of the abortion pill. The Fifth Circuit held in August that the doctors’ challenge to the 2000 approval was untimely, but agreed that FDA’s loosening of restrictions after 2016 should be undone.
The Fifth Circuit ruled, “In loosening mifepristone’s safety restrictions, FDA failed to address several important concerns about whether the drug would be safe for the women who use it.”
The FDA and abortion pill makers Danco Laboratories argue the doctors have not sufficiently shown they have been harmed. They claim that the Fifth Circuit’s reasoning for granting them standing “would bless any suit by an association of healthcare providers challenging any agency decision that might affect a potential patient.”
Danco Laboratories argues in its brief, “Some anti-depressants can increase risks of suicidal thoughts; some drugs to treat one form of cancer can increase the risk of another; some drugs cause birth defects; the list goes on.”
They also challenge the safety claims, writing that the FDA “comprehensively detailed the evidence supporting each decision, made reasonable predictive judgments based on the data, and explained why the evidence supported labeling changes.”
Members of Congress have filed amicus briefs on both sides of the case. In a brief favoring the FDA filed by 263 members, including Democratic Senate Majority Leader Chuck Schumer, they argue the Fifth Circuit’s decision “threatens the congressionally mandated drug approval process and poses a serious health risk to pregnant individuals by making abortion more difficult to access.”
A separate brief filed by 145 pro-life members of Congress argues the FDA “exceeded its congressionally authorized power” by removing the safety regulations. They wrote, “Mifepristone carries significant risks for women and girls, and the FDA exacerbated these risks by unlawfully deregulating chemical abortion drugs.”
U.S. abortions reached 1 million in 2023, the highest level in a decade, largely due to increased access to the chemical abortion pill following the FDA’s decision to lift the in-person requirement, according to a March report. The FDA’s numbers show one in 25 women who use mifepristone will make a trip to the emergency room, according to the plaintiff’s brief.
Dr. Nancy Wozniak, who serves on the board of the AAPLOG, wrote in a declaration filed as part of the case, “Few people die from chemical abortions because of the excellent care they receive from OBGYN doctors, but the infrequency of deaths conceals the danger that these drugs pose to women and girls—especially when administered without proper supervision.”
As our loyal readers, we encourage you to share your thoughts and opinions on this issue. Let your voice be heard and join the discussion below.

-

 Entertainment2 years ago
Entertainment2 years agoWhoopi Goldberg’s “Wildly Inappropriate” Commentary Forces “The View” into Unscheduled Commercial Break
-

 Entertainment1 year ago
Entertainment1 year ago‘He’s A Pr*ck And F*cking Hates Republicans’: Megyn Kelly Goes Off on Don Lemon
-

 Featured2 years ago
Featured2 years agoUS Advises Citizens to Leave This Country ASAP
-

 Featured2 years ago
Featured2 years agoBenghazi Hero: Hillary Clinton is “One of the Most Disgusting Humans on Earth”
-

 Entertainment1 year ago
Entertainment1 year agoComedy Mourns Legend Richard Lewis: A Heartfelt Farewell
-

 Featured2 years ago
Featured2 years agoFox News Calls Security on Donald Trump Jr. at GOP Debate [Video]
-

 Latest News1 year ago
Latest News1 year agoNude Woman Wields Spiked Club in Daylight Venice Beach Brawl
-

 Latest News1 year ago
Latest News1 year agoSupreme Court Gift: Trump’s Trial Delayed, Election Interference Allegations Linger
Glenn LeMaster
March 28, 2024 at 9:57 am
I’d prefer to offer this pill to any young woman as an alternative to an abortion, or bringing an unwanted child into this screwed up world.